Abstract

Background: The combination of cytotoxic chemotherapy with a BCR::ABL1 tyrosine kinase inhibitor (TKI) has historically been standard of care for patients (pts) with Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL). Ponatinib is 3rd generation TKI with more potent BCR::ABL1 inhibition than earlier-generation TKIs. Ponatinib also suppresses T315I clones, which are responsible for most relapses with other TKIs. Here we present the 6-year follow-up results of hyper-CVAD plus ponatinib for patients with newly diagnosed Ph+ ALL.
Methods: This was a phase 2, single-arm study to evaluate the efficacy and safety of hyper-CVAD with ponatinib in adults with newly diagnosed Ph+ ALL. Pts age 18 years or older with previously untreated Ph+ ALL were eligible; pts who had received 1 to 2 courses of prior chemotherapy, with or without a TKI, were also eligible. Pts were required to have an ECOG performance status of 2 or less, bilirubin <3mg/dl, creatinine <3mg/dl, and normal cardiac function. Pts received hyper-CVAD alternating with high-dose methotrexate and cytarabine for 8 cycles. Initially, ponatinib was given at a dose of 45 mg daily. Due to 2 deaths due to cardiovascular events related to ponatinib, beginning with pt #37, the protocol was amended. After the amendment, ponatinib was administered at a dose of 45 mg for 14 days in cycle 1, then 30mg daily once patients achieved CR and 15mg daily once a complete molecular response (CMR) was achieved. Maintenance therapy was given for 2 years, vincristine, prednisone and ponatinib. Pts received 12 doses of intrathecal chemotherapy with alternating doses of cytarabine and methotrexate.
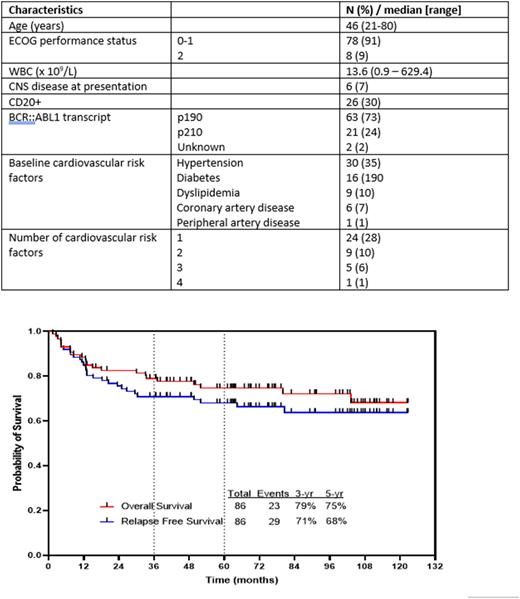
Results: 86 pts were treated. Pts characteristics are summarized in Table 1. The median age was 46 years (range 21-80). Sixty-six pts (77%) had received no prior ALL-directed therapy, and 20 pts (23%) had received 1-2 courses of therapy prior to enrollment. Among these 20 pts, 18 were in CR and 2 had persistent disease at the time of enrollment. Sixty three pts (73%) had a p190 transcript, and 21 (24%) had a p210 transcript.
Among 68 pts with active disease at enrollment, all pts achieved CR. Among 54 pts evaluable for cytogenetic response, all pts achieved complete cytogenetic response, including 50 pts (93%) after 1 cycle. Among 85 pts evaluable for molecular response, 83 pts (98%) achieved at least a major molecular response and 73 pts (86%) achieved a CMR. No pts died in the first 4 weeks of therapy.
After a median follow up of 75 months (range, 16-123+ months), the 5-year overall survival (OS) and relapse-free survival rates were 75% and 68%, respectively (Figure 1). Twenty pts (23%) underwent stem cell transplantation (SCT) in first remission (5 of whom relapsed post SCT and 1 of whom died from post-SCT complications), 10 pts (12%) died in CR, 13 pts (19%) relapsed without SCT, and 43 pts (50%) are in ongoing remission without SCT. Of the relapsed pts, 5 were on ponatinib at the time of relapse, 2 of whom acquired an E255K kinase domain mutation at relapse; and 5 were on imatinib or dasatinib at the time of relapse, 3 of whom acquired a T315I kinase domain mutation. Among the 10 deaths in CR, 2 were treatment-related (both due to myocardial infarction before the amendment to implement lower doses of ponatinib). In a 8-month landmark analysis, the 5-year OS for pts without ASCT and with ASCT were 87% and 70%, respectively (p =0.09). Among the pts who are in ongoing remission without ASCT, 3 received subsequent therapy for MRD-positive disease.
The most common grade ≥3 AE was infections and/or febrile neutropenia, seen in 82 pts (95%). Other grade ≥3 AEs including elevated transaminases in 25 pts (29%), increased amylase and/or lipase in 15 (17%), hyperbilirubinemia in 13 pts (15%), and clinical pancreatitis in 13 pts (15%). Five pts (6%) had a grade ≥3 venous thrombotic event, and 5 pts (6%) had a grade ≥3 arterial cardiovascular event. Two patients died from ponatinib-related cardiovascular complications, both from MI during cycle 2.
Conclusion: The combination of hyper-CVAD and ponatinib results deep and durable remissions in pts with newly diagnosed Ph+ ALL. The overall CMR rate was 86% and the 5-year OS was 75%, which compares favorably with regimens using first- or second-generation TKIs in this population. The combination was safe; the use of lower doses of ponatinib maintained efficacy and mitigated the risk of ponatinib-related toxicity.
Disclosures
Jabbour:Adaptive Biotechnologies: Other: Advisory Role, Research Funding; Genentech: Other: Advisory Role, Research Funding; Bristol Myers Squibb: Other: Advisory Role, Research Funding; Amgen: Other: Advisory Role, Research Funding; Pfizer: Other: Advisory Role, Research Funding; AbbVie: Other: Advisory Role, Research Funding; Spectrum: Research Funding; Takeda: Other: Advisory Role, Research Funding. Short:Astellas: Research Funding; Pfizer: Consultancy; Amgen: Consultancy, Honoraria; Takeda Oncology: Consultancy, Research Funding; AstraZeneca: Consultancy; Stemline Therapeutics: Research Funding; Novartis: Consultancy. Sasaki:Pfizer: Membership on an entity's Board of Directors or advisory committees; Otsuka Pharmaceuticals: Honoraria; Daiichi-Sankyo: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Ravandi:Amgen: Honoraria, Research Funding; Novartis: Consultancy; Biomea Fusion, Inc.: Research Funding; Xencor: Research Funding; Prelude: Research Funding; Amgen: Honoraria, Research Funding; BMS/Celgene: Consultancy, Honoraria, Research Funding; Astellas: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy; Abbvie: Consultancy, Honoraria, Research Funding; Astex/Taiho: Membership on an entity's Board of Directors or advisory committees, Research Funding; Syos: Consultancy, Honoraria, Research Funding. Jain:Incyte Corporation: Research Funding; Mingsight: Research Funding; AbbVie: Consultancy, Honoraria, Other: Travel Support, Research Funding; TransThera Sciences: Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria, Other: Travel Support, Research Funding; Takeda: Research Funding; Medisix: Research Funding; Loxo Oncology: Research Funding; AstraZeneca: Consultancy, Honoraria, Other: Travel Support, Research Funding; BMS: Consultancy, Honoraria, Other: Travel Support, Research Funding; Novalgen: Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding; Servier Pharmaceuticals LLC: Research Funding; Dialectic Therapeutics: Research Funding; Newave: Research Funding; Fate Therapeutics: Research Funding; Aprea Therapeutics: Research Funding; Pfizer: Research Funding; Cellectis: Honoraria, Research Funding; ADC Therapeutics: Research Funding; Precision Biosciences: Consultancy, Honoraria, Other: Travel Support, Research Funding; Pharmacyclics, Inc.: Consultancy, Honoraria, Other: Travel Support, Research Funding; Genentech, Inc.: Consultancy, Honoraria, Other: Travel Support, Research Funding; Janssen Pharmaceuticals, Inc.: Consultancy, Honoraria, Other: Travel Support; Beigene: Honoraria; Cellectis: Honoraria, Research Funding; TG Therapeutics: Honoraria; MEI Pharma: Honoraria; Ipsen: Honoraria; CareDx: Honoraria. Daver:Agios, Celgene, SOBI and STAR Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kartos and Jazz Pharmaceuticals: Other: Data monitoring committee member; Karyopham Therapeutics and Newave Pharmaceutical: Research Funding; Astellas, AbbVie, Genentech, Daiichi-Sankyo, Novartis, Jazz, Amgen, Servier, Karyopharm, Trovagene, Trillium, Syndax, Gilead, Pfizer, Bristol Myers Squibb, Kite, Actinium, Arog, Immunogen, Arcellx, and Shattuck: Consultancy, Other: Advisory Role; Astellas, AbbVie, Genentech, Daiichi-Sankyo, Gilead, Immunogen, Pfizer, Bristol Myers Squibb, Trovagene, Servier, Novimmune, Incyte, Hanmi, Fate, Amgen, Kite, Novartis, Astex, KAHR, Shattuck, Sobi, Glycomimetics, Trillium: Research Funding. Pemmaraju:stemline: Consultancy; abbvie: Consultancy; immunogen: Consultancy; mustangbio: Research Funding; incyte: Consultancy; novartis: Research Funding; pacylex: Consultancy, Research Funding; samus: Research Funding; daiichi sankyo: Research Funding; cellectis: Research Funding; cellularity: Research Funding. Yilmaz:Pfizer: Research Funding; Daiichi-Sankyo: Research Funding. Kadia:PinotBio: Consultancy; Servier: Consultancy; cellenkos: Research Funding; Genfleet: Research Funding; Astellas: Research Funding; cyclacel: Research Funding; Amgen: Research Funding; Ascentage: Research Funding; BMS: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; AstraZeneca: Research Funding; Iterion: Research Funding; Glycomimetics: Research Funding; Pfizer: Research Funding; Regeneron: Research Funding; Agios: Consultancy; Abbvie: Consultancy, Research Funding; Delta-Fly: Research Funding; Novartis: Consultancy; JAZZ: Consultancy, Research Funding; Astex: Honoraria. Garcia-Manero:Genentech: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Curis: Honoraria, Research Funding; Acceleron Pharma: Consultancy; Astex: Consultancy, Honoraria, Research Funding; Aprea: Honoraria; Gilead Sciences: Research Funding. DiNardo:Jazz: Honoraria; LOXO: Research Funding; Servier: Consultancy, Honoraria, Research Funding; Foghorn: Honoraria, Research Funding; ImmuneOnc: Honoraria, Research Funding; GenMab: Membership on an entity's Board of Directors or advisory committees; Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Honoraria, Research Funding; Takeda: Honoraria; Astex: Research Funding; Gilead: Honoraria; AbbVie: Consultancy, Research Funding; Cleave: Research Funding; Novartis: Honoraria; Astellas: Honoraria; Bluebird Bio: Honoraria; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Kura: Honoraria, Membership on an entity's Board of Directors or advisory committees; Forma: Research Funding. Konopleva:Stemline Therapeutics, F. Hoffman La-Roche; Janssen: Membership on an entity's Board of Directors or advisory committees; AbbVie, Genentech, F. Hoffman La-Roche, Stemline Therapeutics, Amgen, Forty-Seven, Kisoji; Janssen: Consultancy; Stocks, Reata Pharmaceuticals: Current equity holder in publicly-traded company; Reata Pharmaceuticals, Novartis and Eli Lilly: Patents & Royalties; AbbVie, Genentech, F. Hoffman La-Roche, Eli Lilly, Cellectis, Calithera, Ablynx, Stemline Therapeutics, Agios, Ascentage, Astra Zeneca; Rafael Pharmaceutical; Sanofi, Forty-Seven: Research Funding; Forty-Seven; F. Hoffman LaRoche: Honoraria. Wierda:Genzyme: Consultancy; Sanofi: Consultancy; Karyopharm: Research Funding; Xencor: Research Funding; Juno: Research Funding; Sunesis: Research Funding; Pharmacyclics LLC: Research Funding; Oncternal Therapeutics, Inc.: Research Funding; Miragen: Research Funding; GSK/Novartis: Research Funding; Genentech: Research Funding; Gilead Sciences: Research Funding; Janssen: Research Funding; Loxo Oncology, Inc./Lilly: Research Funding; Kite, a Gilead Company: Research Funding; Cyclacel: Research Funding; Bristol Meyers Squibb (Juno and Celgene): Research Funding; AstraZeneca/Acerta Pharma. Inc.: Research Funding; AbbVie: Research Funding. Kantarjian:AbbVie: Honoraria, Research Funding; Ascentage: Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas Health: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Research Funding; Jazz Pharmaceuticals: Research Funding; Daiichi-Sankyo: Consultancy, Research Funding; Novartis: Honoraria, Research Funding; ImmunoGen: Research Funding; Ipsen Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; KAHR Medical Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees; NOVA Research: Honoraria; Pfizer: Honoraria, Research Funding; Takeda: Honoraria.
OffLabel Disclosure:
ponatinib for frontline use in Ph+ ALL
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.